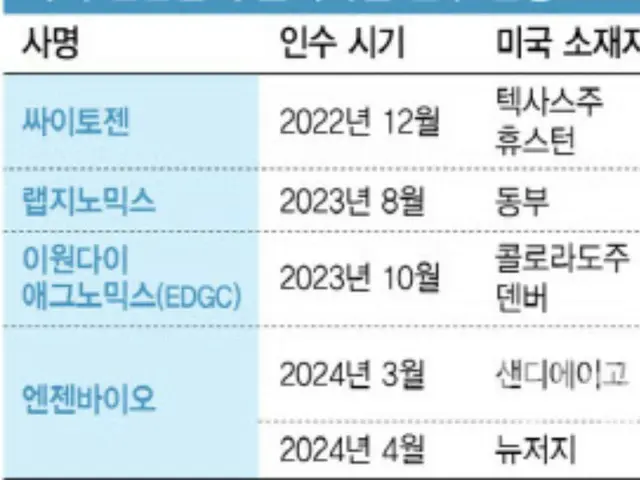
This will allow CytoGen to enter the U.S. market without needing to obtain approval from the Food and Drug Administration (FDA). In the case of CytoGen, Expertox, a U.S. company it acquired in 2022, is expected to account for 70% of the company's total sales in 2023.
The company's sales for 2023 are expected to reach 3.2 billion won (US$3.6 million), a four-fold increase from the previous year.
On the other hand, the company's net loss for the past three years has remained stable, from 13.3 billion won (approximately 1.5 billion yen) in 2021 to 16.2 billion won (approximately 1.8 billion yen) in 2023.
Although sales were secured, there is still room for improvement in terms of profit and loss. An industry insider said, "In the United States, the number of CLIA-compliant laboratories has increased rapidly since the COVID-19 pandemic, but the business conditions for these laboratories have not been good since the pandemic subsided."
Furthermore, on April 29th (local time), the FDA revised its regulations, announcing a rule that would allow non-regulated tests (LDTs), which it had been overseeing, to be in vitro diagnostic tests. This will allow CLIA-compliant laboratories to
The acquisition would also eliminate the expected benefit of not requiring FDA approval for diagnostic products. However, there are fears that the application of this rule in the U.S. will be met with opposition from the industry.
Industry officials predict legal action will be taken to challenge the final rule.
2024/05/09 08:48 KST
Copyrights(C) Edaily wowkorea.jp 101